Practice isotope calculations 1 answer key – Welcome to the ultimate guide to practice isotope calculations! In this comprehensive resource, we delve into the fascinating world of isotopes, empowering you with a deep understanding of their applications and significance. Through a series of engaging practice problems and expert explanations, you’ll master the art of isotope calculations, unlocking a wealth of knowledge in various fields.
Our journey begins with an exploration of the fundamental concepts of isotopes and their atomic structures. We’ll uncover the diverse types of isotopes and their practical applications, providing you with a solid foundation for understanding their role in various scientific disciplines.
1. Isotope Calculations: Practice Isotope Calculations 1 Answer Key
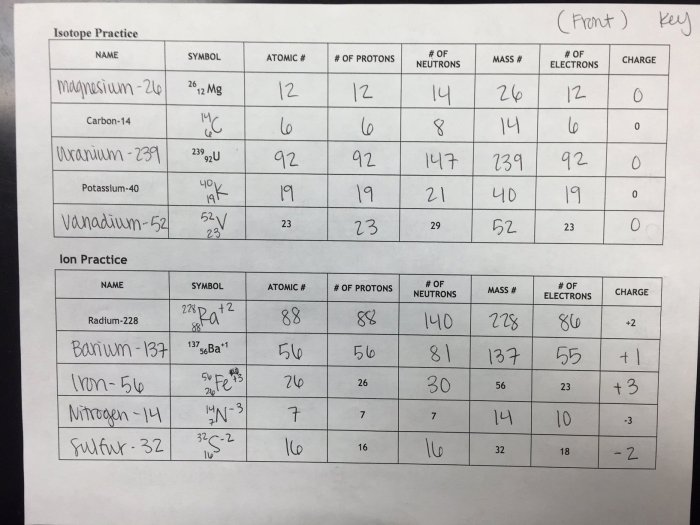
Isotopes are variations of an element that have the same atomic number but different mass numbers. They arise due to differences in the number of neutrons in the atomic nucleus.
The atomic number determines the number of protons in the nucleus, which defines the element’s chemical properties. The mass number is the sum of the number of protons and neutrons.
Isotopes can be stable or radioactive. Stable isotopes do not undergo nuclear decay, while radioactive isotopes decay over time, emitting particles and energy.
Different Types of Isotopes
- Stable isotopes: Do not undergo radioactive decay and are commonly used in various applications.
- Radioactive isotopes: Decay over time, emitting particles and energy, and have applications in medicine, dating techniques, and industry.
Applications of Isotopes
Isotopes have a wide range of applications in various fields:
- Medicine: Diagnostic imaging (e.g., PET scans), cancer treatment (e.g., radiotherapy).
- Environmental studies: Tracing pollutants, dating fossils.
- Industry: Quality control, material analysis.
2. Practice Problems
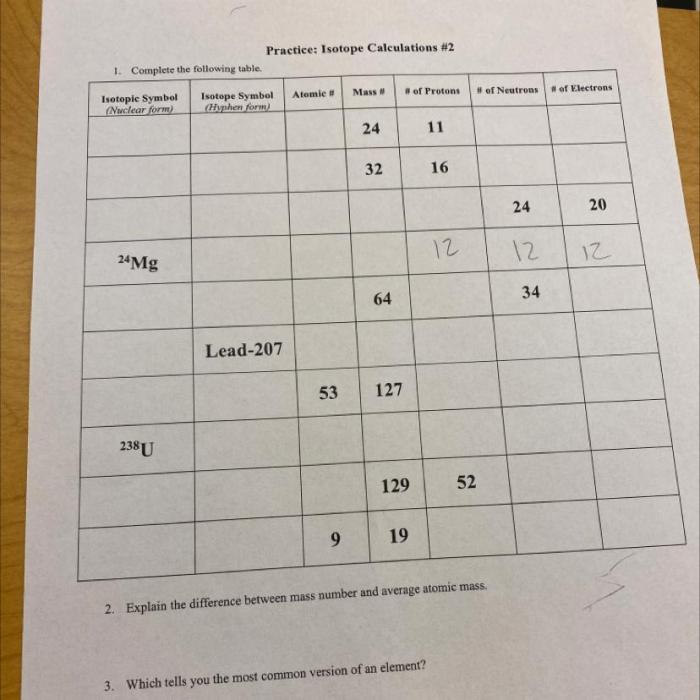
To practice isotope calculations, let’s consider the following table:
| Isotope | Mass Number | Atomic Number | Abundance (%) |
|---|---|---|---|
| 12C | 12 | 6 | 98.93 |
| 13C | 13 | 6 | 1.07 |
Practice Problem: Finding Average Atomic Mass
Calculate the average atomic mass of carbon using the data in the table.
Solution:
Average atomic mass = (Mass number of 12C x Abundance of 12C) + (Mass number of 13C x Abundance of 13C)
= (12 x 0.9893) + (13 x 0.0107)
= 12.01 amu
3. Advanced Isotope Calculations
Radioactive isotopes decay at specific rates, which can be used for dating techniques:
- Carbon dating: Used to date organic materials up to 50,000 years old.
- Potassium-argon dating: Used to date rocks and minerals up to several billion years old.
Medical Applications of Radioactive Isotopes, Practice isotope calculations 1 answer key
- Diagnostic imaging: PET scans, bone scans.
- Cancer therapy: Radiotherapy, targeted radionuclide therapy.
Questions and Answers
What are isotopes?
Isotopes are atoms of the same element that have the same atomic number but different mass numbers.
How are isotopes used in medicine?
Isotopes are used in medicine for diagnostic imaging, such as PET scans, and cancer treatment, such as radiation therapy.
How are isotopes used in environmental studies?
Isotopes are used in environmental studies to trace pollutants and date fossils.