Ethyl alcohol likely exhibits more hydrogen bonding than water, a concept that unveils a captivating realm of intermolecular interactions. This discourse delves into the intricacies of hydrogen bonding, contrasting the molecular characteristics of ethyl alcohol and water to illuminate their distinct hydrogen bonding strengths.
As we unravel the interplay between molecular structure and hydrogen bonding, we gain insights into the remarkable properties and applications of these ubiquitous substances.
Hydrogen Bonding
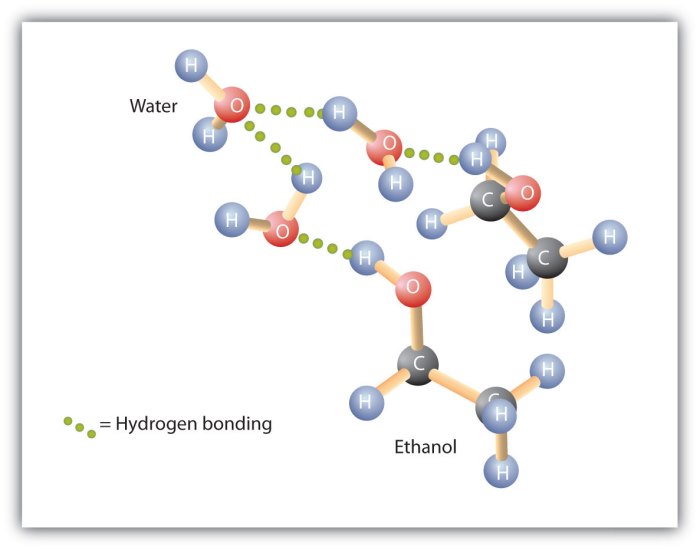
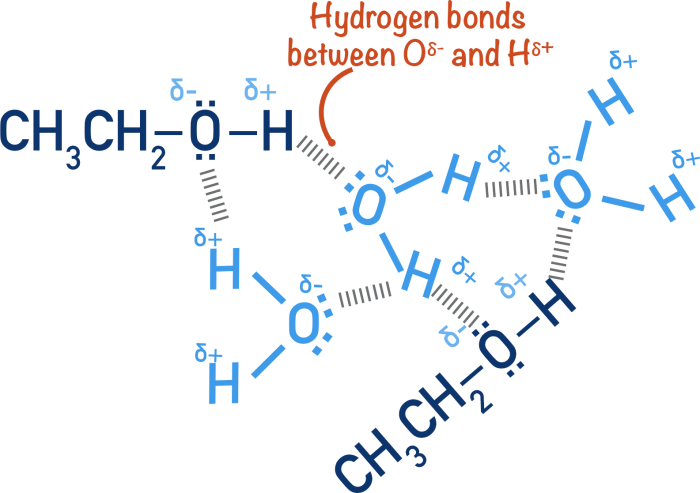
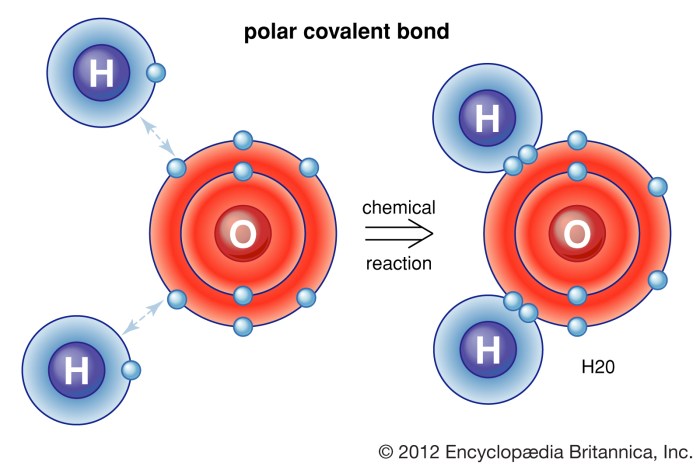
Hydrogen bonding is a dipole-dipole interaction that occurs between a hydrogen atom covalently bonded to a highly electronegative atom (such as oxygen, nitrogen, or fluorine) and another electronegative atom. The hydrogen atom has a partial positive charge due to the electronegativity difference, and the electronegative atom has a partial negative charge.
This allows the hydrogen atom to form a weak bond with the electronegative atom, which is known as a hydrogen bond.
Ethyl Alcohol vs. Water
Ethyl alcohol (C2H5OH) and water (H2O) are both polar molecules with hydroxyl groups (-OH). However, ethyl alcohol has a longer carbon chain and a lower molecular weight (46 g/mol) than water (18 g/mol). Ethyl alcohol is also less polar than water due to the presence of the nonpolar carbon chain.
The hydrogen bonds in ethyl alcohol are weaker than the hydrogen bonds in water. This is because the electronegativity of the oxygen atom in ethyl alcohol is lower than the electronegativity of the oxygen atom in water. As a result, the partial positive charge on the hydrogen atom in ethyl alcohol is weaker than the partial positive charge on the hydrogen atom in water.
Hydrogen Bonding Interactions, Ethyl alcohol likely exhibits more hydrogen bonding than water
The strength of a hydrogen bond depends on the number of hydrogen atoms involved and the electronegativity of the atoms involved. The more hydrogen atoms involved, the stronger the hydrogen bond. The more electronegative the atoms involved, the stronger the hydrogen bond.
In ethyl alcohol, there is only one hydrogen atom involved in hydrogen bonding. In water, there are two hydrogen atoms involved in hydrogen bonding. Therefore, the hydrogen bonds in water are stronger than the hydrogen bonds in ethyl alcohol.
Applications of Hydrogen Bonding
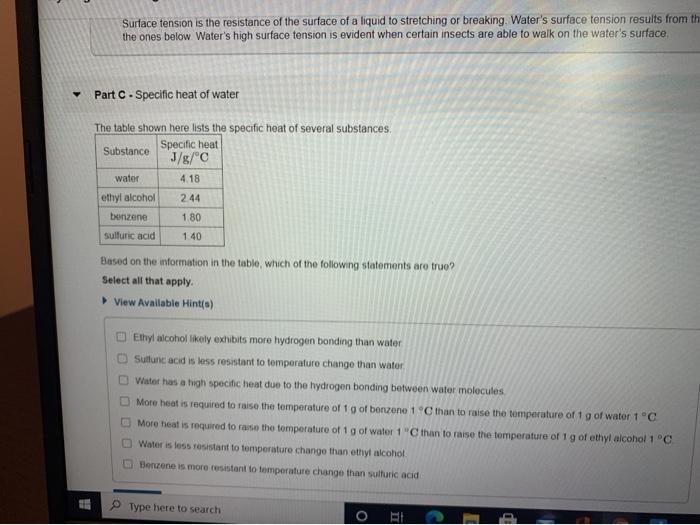
Hydrogen bonding plays an important role in the properties of ethyl alcohol and water. Hydrogen bonding is responsible for the high boiling point of water (100 °C) compared to ethyl alcohol (78 °C). Hydrogen bonding also affects the solubility of ethyl alcohol and water.
Ethyl alcohol is more soluble in water than in nonpolar solvents because of its ability to form hydrogen bonds with water molecules.
Hydrogen bonding is also used in a variety of industrial and biological applications. For example, hydrogen bonding is used to stabilize proteins and DNA. Hydrogen bonding is also used in the production of polymers and other materials.
Question Bank: Ethyl Alcohol Likely Exhibits More Hydrogen Bonding Than Water
What factors contribute to the stronger hydrogen bonding in ethyl alcohol compared to water?
Ethyl alcohol possesses a larger molecular weight and a more polar molecular structure than water. These attributes enhance the electronegativity of the oxygen atom involved in hydrogen bonding, strengthening the electrostatic interactions between molecules.
How does hydrogen bonding impact the physical properties of ethyl alcohol and water?
Hydrogen bonding influences various physical properties, including boiling point, solubility, and viscosity. Ethyl alcohol’s stronger hydrogen bonding results in a higher boiling point, lower solubility in nonpolar solvents, and increased viscosity compared to water.
What are some practical applications of hydrogen bonding in ethyl alcohol?
Hydrogen bonding plays a crucial role in the use of ethyl alcohol as a solvent, disinfectant, and fuel additive. Its ability to form hydrogen bonds with various substances enables its effectiveness in these applications.