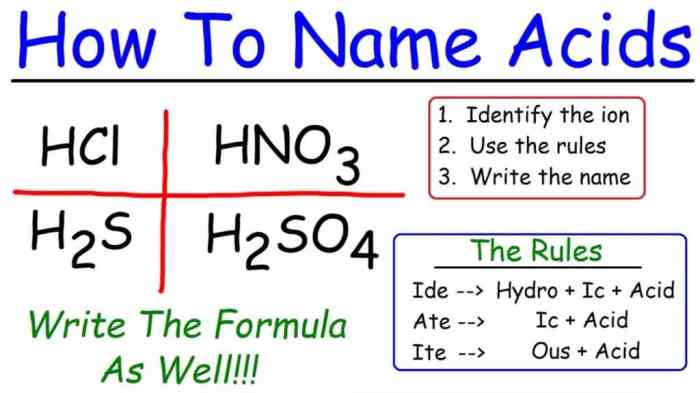
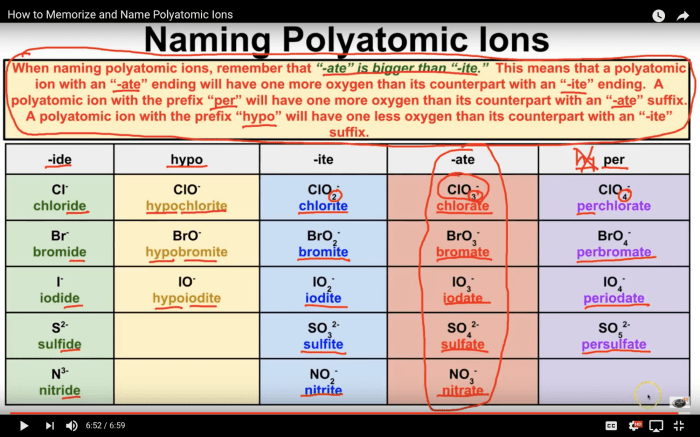
Welcome to our in-depth exploration of predicting and naming polyatomic ionic compounds! This guide, encompassing the Predicting and Naming Polyatomic Ionic Compounds Worksheet Answers, is meticulously crafted to unravel the complexities of this fascinating chemical concept. Prepare to delve into a realm where ions dance and formulas unfold, revealing the secrets of molecular nomenclature.
As we embark on this journey, we will uncover the essence of polyatomic ions, unraveling their unique characteristics and diverse identities. We will master the art of predicting the names of these intricate compounds, deciphering the rules that govern their nomenclature.
Additionally, we will delve into the intricacies of writing their formulas, empowering you with the ability to represent these ionic entities with precision.
Polyatomic Ions
Polyatomic ions are ions that are composed of more than one atom. They are typically formed when a metal loses or gains electrons to form a positively or negatively charged ion, and then combines with a non-metal to form a polyatomic ion.
Some common examples of polyatomic ions include:
- Ammonium (NH 4+)
- Hydroxide (OH –)
- Nitrate (NO 3–)
- Sulfate (SO 42-)
- Carbonate (CO 32-)
Polyatomic ions can be identified in chemical formulas by their characteristic charge. For example, the ammonium ion always has a charge of +1, the hydroxide ion always has a charge of -1, and so on.
Predicting the Names of Polyatomic Ionic Compounds
The names of polyatomic ionic compounds are based on the names of the ions that they contain. The first part of the name is the name of the cation (the positively charged ion), and the second part of the name is the name of the anion (the negatively charged ion).
To predict the name of a polyatomic ionic compound, follow these steps:
- Identify the cation and anion in the compound.
- Write the name of the cation first, followed by the name of the anion.
- Add the suffix “-ide” to the name of the anion.
For example, the compound NaCl is composed of the sodium cation (Na +) and the chloride anion (Cl –). The name of the compound is therefore sodium chloride.
Writing the Formulas of Polyatomic Ionic Compounds: Predicting And Naming Polyatomic Ionic Compounds Worksheet Answers
The formulas of polyatomic ionic compounds are based on the charges of the ions that they contain. The formula of a polyatomic ionic compound must be neutral, meaning that the total positive charge of the cations must be equal to the total negative charge of the anions.
To write the formula of a polyatomic ionic compound, follow these steps:
- Identify the cation and anion in the compound.
- Write the symbol of the cation first, followed by the symbol of the anion.
- Balance the charges of the ions by using subscripts.
For example, the compound NaCl is composed of the sodium cation (Na +) and the chloride anion (Cl –). The formula of the compound is therefore NaCl.
Worksheet Answers
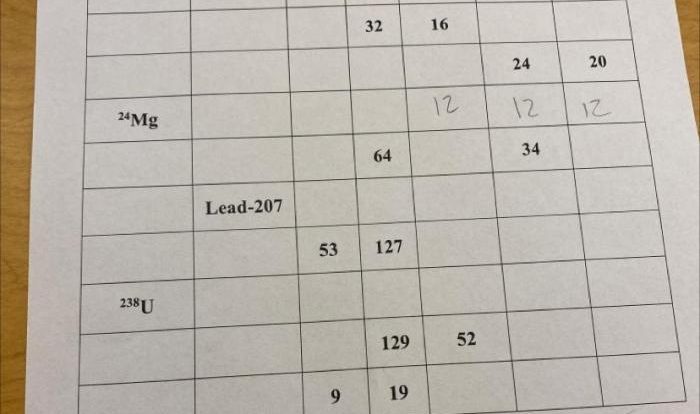
1. What is a polyatomic ion?
A polyatomic ion is an ion that is composed of more than one atom.
2. What are some common examples of polyatomic ions?
Some common examples of polyatomic ions include ammonium (NH 4+), hydroxide (OH –), nitrate (NO 3–), sulfate (SO 42-), and carbonate (CO 32-).
3. How can you identify polyatomic ions in chemical formulas?
Polyatomic ions can be identified in chemical formulas by their characteristic charge.
4. How do you predict the name of a polyatomic ionic compound?
To predict the name of a polyatomic ionic compound, follow these steps:
- Identify the cation and anion in the compound.
- Write the name of the cation first, followed by the name of the anion.
- Add the suffix “-ide” to the name of the anion.
5. How do you write the formula of a polyatomic ionic compound?
To write the formula of a polyatomic ionic compound, follow these steps:
- Identify the cation and anion in the compound.
- Write the symbol of the cation first, followed by the symbol of the anion.
- Balance the charges of the ions by using subscripts.
FAQ Guide
What are polyatomic ions?
Polyatomic ions are groups of atoms that carry an electric charge and behave as a single unit within a compound.
How do I predict the name of a polyatomic ionic compound?
To predict the name of a polyatomic ionic compound, first identify the metal and non-metal ions involved. The metal ion is typically named first, followed by the polyatomic ion, which ends in -ide.
How do I write the formula of a polyatomic ionic compound?
To write the formula of a polyatomic ionic compound, first determine the charges of the metal and polyatomic ions. Then, balance the charges by adjusting the subscripts of the ions. Finally, write the formula with the metal ion first, followed by the polyatomic ion.